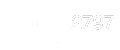Certified partner for CROs
In addition to creating proprietary medical software applications, Medimaps operates as a trusted consulting and contract research organization (CRO) for pharmaceutical organizations looking to integrate bone microarchitecture assessment in their discovery and clinical studies.
Since 2019, Medimaps is a certified and trusted partner of the world’s leading CROs and top global pharmaceutical companies. Our centralized TBS analysis service has:
- Supported over 12 clinical trials with accurate and reliable TBS analysis.
- Provided actionable insights to enhance clinical trial outcomes and support regulatory submissions.
- Enabled precise assessment of bone health, contributing to the development and validation of innovative therapies.
What we offer
Trabecular Bone Score research analysis
Trabecular Bone Score calibration
Trabecular Bone Score report analysis
Texture Research Investigation Platform (TRIP)
Enhancing clinical trials with Trabecular Bone Score (TBS)
Including bone microarchitecture as a clinical marker can provide unique and complementary insights into treatment effects. In particular, this could lead to improved patient stratification and a more accurate evaluation of treatment efficacy. Medimaps has a proven success record of working with clinical trial entities across the product lifecycle.
Benefits
- Discovery studies: For research purpose only, our Texture Research Investigation Platform (TRIP) enables customers to analyze the trabecular bone texture at different skeletal sites (e.g. spine, hip, proximal and distal femur, knee, tibia, humerus, shoulders) and from different image modalities. TRIP relies on the experimental variogram algorithm, as implemented in TBS iNsight.
- Phase II trials: To determine the efficacy and safety of the investigational product.
- Phase III trials: To confirm the investigational product’s efficacy and to monitor side effects in a larger group of patients. Including TBS can help in providing comprehensive evidence of a product’s benefits and risks, contributing to support regulatory approval.
- Post-market surveillance: Following commercialization, monitoring changes in bone microarchitecture can help assess long-term safety and efficacy, ensuring ongoing and optimal therapeutic outcomes.
They trust us
- Agnovos
- Amgen
- Astellas
- Eli-Lilly
- Takeda
- Radius Pharma
- Clario
- Calyx
- Medpace
Evidence-based value of TBS for secondary causes of osteoporosis
Published evidence highlights the value of TBS in clinical trials across various fields where bone health is compromised due to disease pathophysiology or therapeutic treatment.
Degraded TBS is commonly observed in diseases associated with osteoporosis and is linked to an increased risk of fracture, even when bone mineral density (BMD) is normal.
TBS independently predicts fracture risk in patients with diabetes, rheumatological diseases, chronic kidney disease, and those undergoing long-term glucocorticoid therapy or treatment with aromatase inhibitors – conditions known to affect bone integrity.
Additionally, TBS is relatively insensitive to certain spinal changes, such as the presence of osteophytes and syndesmophytes, which can erroneously elevate BMD assessments.